Listed below is a set of representative data similar to what you will be using in your Pre-Lab, Quiz and Lab for the experiment on Rate Laws. After reviewing this material, fill in the spaces below for the concentrations, rates and, ultimately, rate law. The solutions will be posted at a later time so check back on the website!
Reaction: C2H2Cl2 + I2 -> C2H2Cl2I2
I. Preparing to Measure Solution Volumes
concentration of C2H2Cl2solution, mol/L 0. 264 M .
concentration of I2 solution, mol/L 0.0128 M .
room temperature, °C 22.2oC .
II. Determining the Effect of Reactant Concentrations on the Reaction Rate:
determination
number |
volume of C2H2Cl2
solution, mL |
volume of I2
solution, mL |
volume of
water, mL |
1 |
1.00 |
5.00 |
6.00 |
|
|
|
|
2 |
2.00 |
5.00 |
5.00 |
|
|
|
|
3 |
1.00 |
10.00 |
1.00 |
|
|
|
|
Using the calculations and data provided, determine the orders, x and y and calculate the rate constant, k. Place the values in the spaces below and then submit your answers for credit.
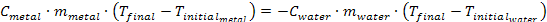
x =
y =
k =

